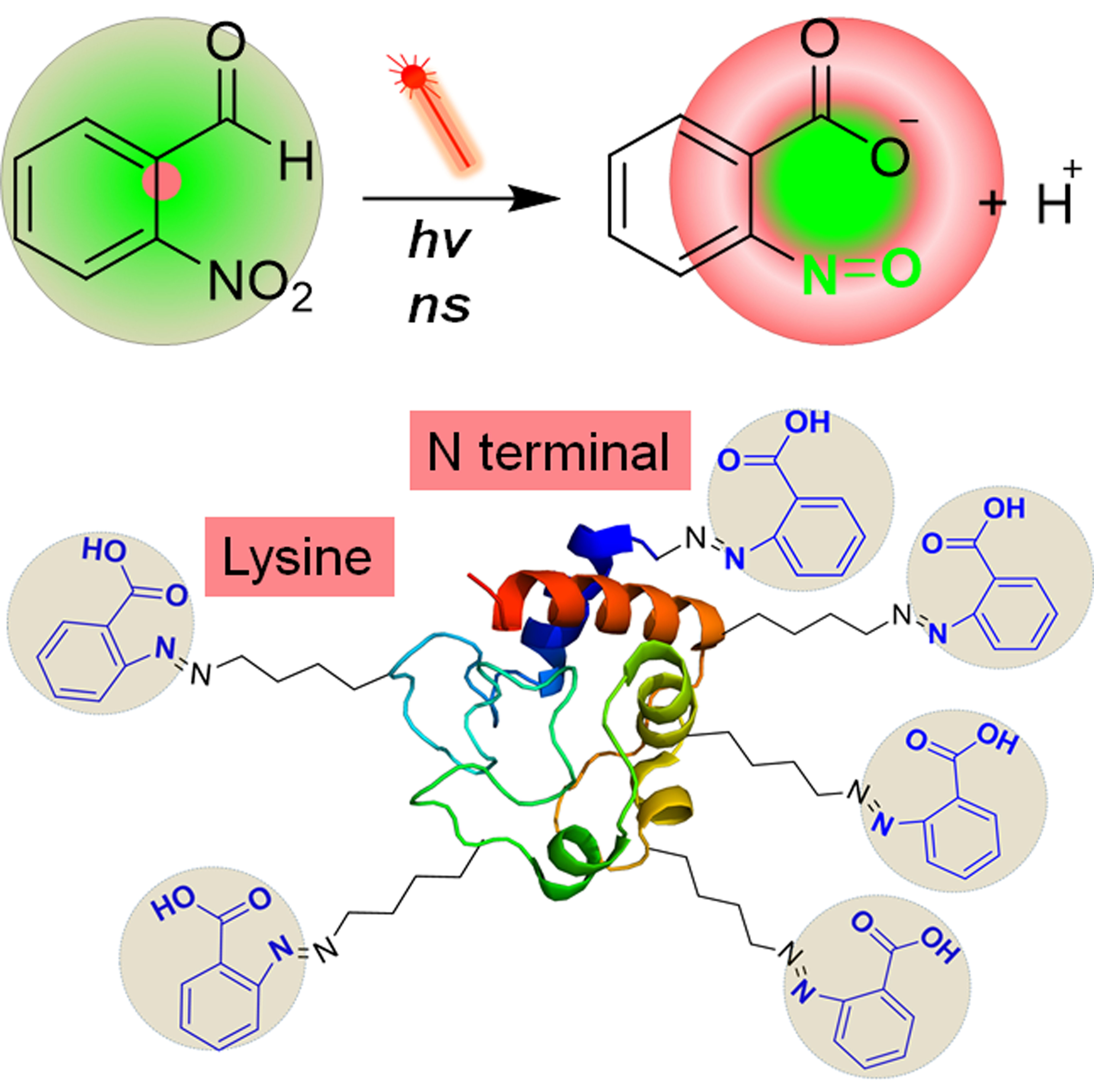
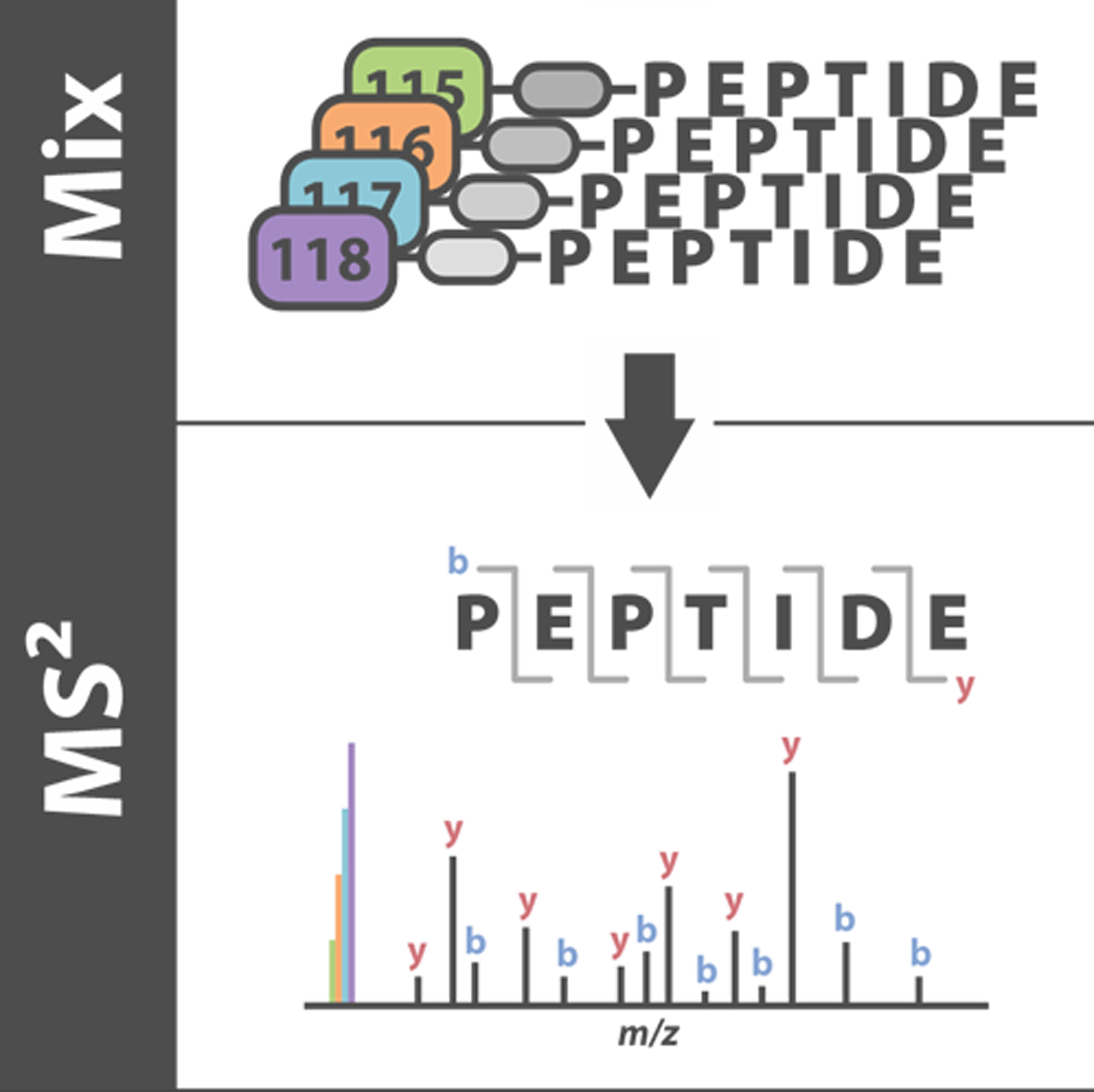
To ultimately provide molecular basis and next-generation therapeutics for human disease, Dr. Gongyu Li has long-standing research interests in the development and application of ultrafast separation-equipped mass spectrometry (MS) techniques capable of: 1) in situ identification, 2) rapid structural elucidation and 3) expedited functional interrogation of human disease-linked bioactive molecules, including differentially folded proteins/ neuropeptides/metabolites, directly from living cells or even fresh tissues. The most significant challenge is, however, how to remove intracellular and extracellular matrices surrounding target biomolecules without external perturbations on their native biological states, where the separation time scale should be as short as possible to maintain the biologically relevant structural information. To solve this problem, during his PhD career, Dr. Li has involved in the development of an ultrafast separation regime and integrated into nanospray, generating a new versatile ambient MS method (Li et al. Anal. Chem. 2016, 88, 10860-10866), and has demonstrated its utility for several protein systems directly from living cells (Li et al. Anal. Chem. 2018, 90, 3409-3415). In addition to contribution to the Ultrafast MS identification of native proteins, Gongyu also devoted himself in gas-phase protein structural interrogations, such as the development of a new regime for ion mobility-mass spectrometry (IM-MS)-based protein structural manipulations (Li et al. Anal. Chem. 2018, 90, 7997-8001). Dr. Li’s postdoc research work is focused on further expanding MS methodologies for better identification and structural interrogation of human health-linked bioactive molecules, ranging from native proteins, neuropeptides to metabolites. To this end, Gongyu has already developed a new millisecond ion mobility separation platform to investigate chiral effect of amyloid beta peptide and its implications in Alzheimer’s disease therapeutic development (Li et al. Nat. Commun. 2019, 10, 5038) and a nanosecond photochemical click chemistry method for rapid neuropeptide and protein structural analysis (Li et al. Nat. Commun. 2019, 10, 4697, Featured by Nature Communications Editors HIGHLIGHTS). By Feburary 2022, Dr. Li’s research efforts, overall, have generated 4 grants, 7 fellowships/awards, > 10 oral presentations and 29 scientific publications, including 2 Nat. Commun., 6 Anal. Chem., 1 Chem. Sci. and 1 Trends Anal. Chem. papers, featuring with 550+ citations.
南开大学李功玉课题组LimsLab@NKU的研究方向为蛋白结构离子淌度质谱分析。针对结构质谱分析存在的原位取样困难、脱溶剂破坏蛋白结构以及结构分辨率不足等技术问题, 李功玉课题组整合了针对细胞内完整蛋白的在线感应电穿孔原位释放技术与毫秒微电泳快速分离技术,开发了基于纳秒光化学点击反应的快速捕获蛋白高级结构策略, 并发展了“仪器非依赖型手性拆分”高分辨离子淌度质谱分析方法,由此搭建了蛋白质快速检测和无损高分辨结构质谱分析平台,依次在细胞、组织和人类疾病模型水平上开展了系统性研究, 最终在Dalton质量分辨率水平上,快速揭示了“氨基酸手性调控阿尔茨海默症相关蛋白结构和毒性”以及“唾液酸化修饰调控转铁蛋白高级结构”的分子基础。 为解决传统蛋白质质谱技术在蛋白质相互作用信息、细胞异质性信息以及细胞动态变化信息等 多方面的不足,LimsLab提出原位结构质谱的概念,将整合原位蛋白质质谱、离子淌度结构质谱和靶向蛋白质 组学的优势,开发出一整套针对蛋白结构及蛋白修饰构效关系的超快质谱分析方法,长期目标为实现单个活细胞内单个蛋白质结构的快速质谱分析。LimsLab课题组在蛋白质 质谱方向上有十余年的研究经验,包括质谱基础理论研究、新型快速原位灵敏的质谱分析方法开发以及在 阿尔茨海默症相关蛋白结构研究中的应用研究。LimsLab核心任务是为病原微生物感染机制和早期诊疗提供蛋白质 分子机制水平上的新视角新策略,技术上重点解决蛋白质结构质谱分析时存在的技术难题(比如常规结构质谱技术的环境兼容性问题), 科学上重点关注低丰度蛋白质翻译后修饰(如氨基酸手性突变和唾液酸化等)对蛋白结构和功能的影响。 具体方向包括:(1)原位结构质谱一步检测活细胞内蛋白结构及其相互作用(原位蛋白鉴定);(2)化学结构质谱方法学开发及其 用于蛋白修饰构效关系研究(结构无损分析);(3)手性结构质谱用于人类疾病关键蛋白的手性拆分(功能应用研究)。
李功玉,南开大学化学学院,研究员、博士生导师。入选国家高层次青年人才计划、主持科技部重点研发青年项目。2021年2月加入南开大学化学学院,成立LimsLab课题组,研究方向为大分子结构质谱分析。 以通讯或第一作者发表SCI论文20篇(含2篇Nat. Commun.和 6篇Anal. Chem.)。课题组成立以来,获取可支配经费近千万元,指导3名本科生获批大创项目,3名本科生完成毕业设计,以南开大学为通讯单位发表2篇Anal Chem。 成功组建一支15人的年轻科研队伍,课题组现有研究生8人,本科生3人,科研助理4人,团队组成合理,继续虚位以待,期待您的加入与合作!